Industries
Powering regulated manufacturing with a purpose-built ERP for batch-driven operations,
uncompromising quality, and end-to-end regulatory compliance.
FOOD &
BEVERAGES
Supports FSSAI and HACCP requirements through controlled, traceable batch processing.
PAINT &
COATINGS
Safely manage formulas, control costs, and meet regulatory requirements—without complexity.
PHARMA
MANUFACTURING
From formulation to finished product—every step is controlled, compliant, and audit-ready.
SPECIALTY
CHEMICALS
Manage formulas, drive innovation, handle inventory, and execute compliant production.
Personal Care &
Cosmetics
Right formulation, high quality, and multiple packaging options to beautify business growth.
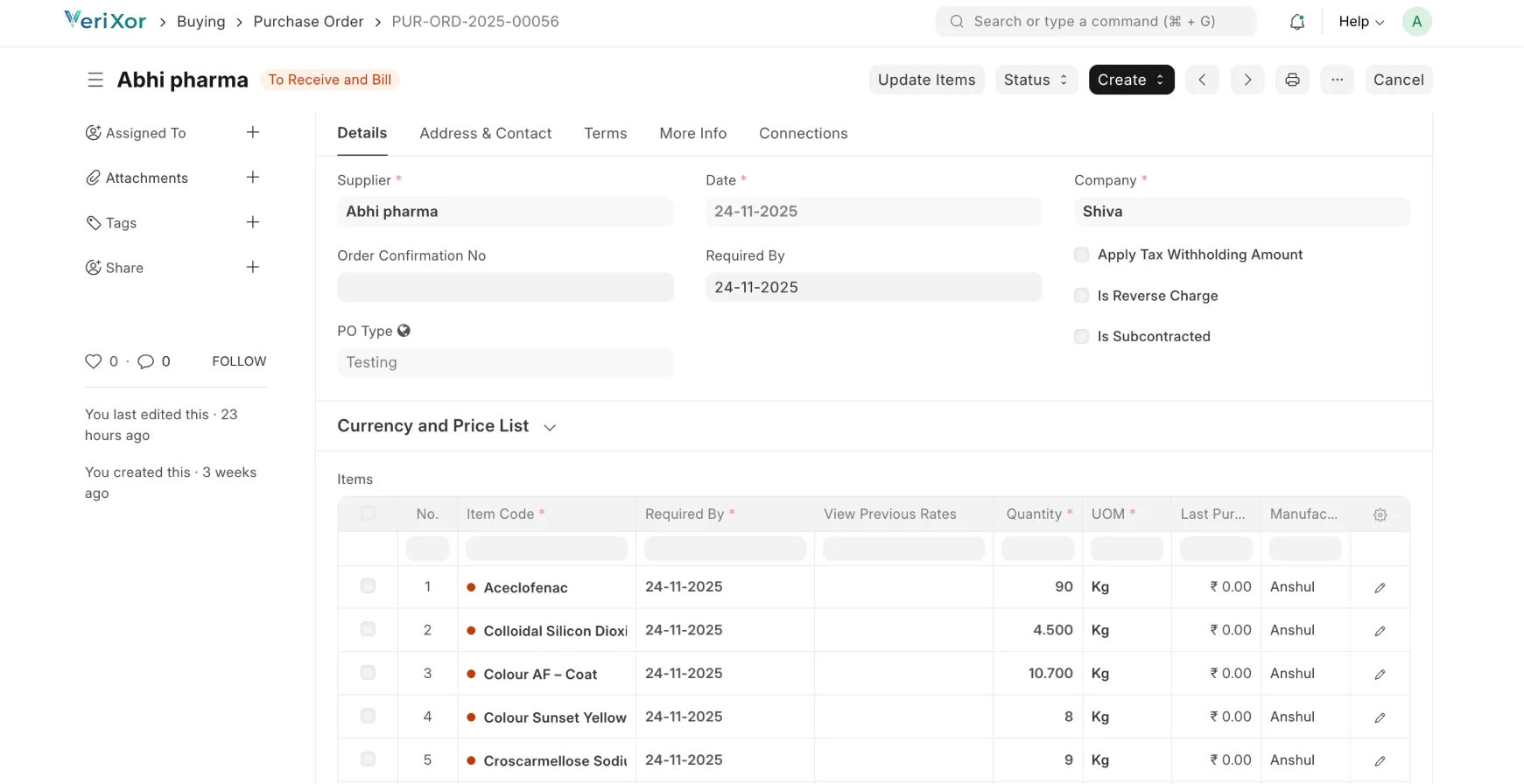
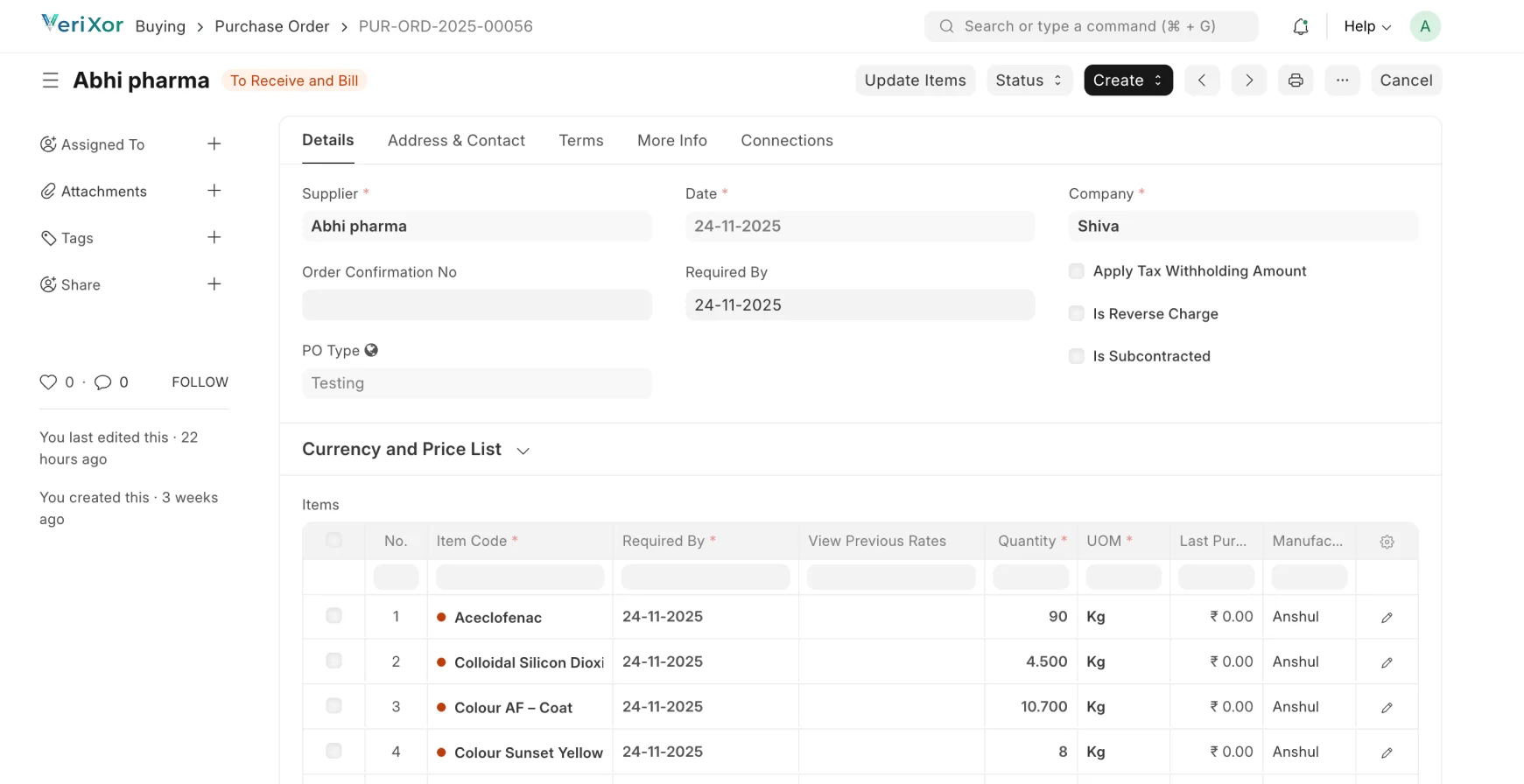
Streamlined, Traceable & Compliant Pharma Fulfillment
Manage formulation, production, quality, and dispatch with full FDA,
cGMP & 21 CFR Part 11 compliance.
Order Generation
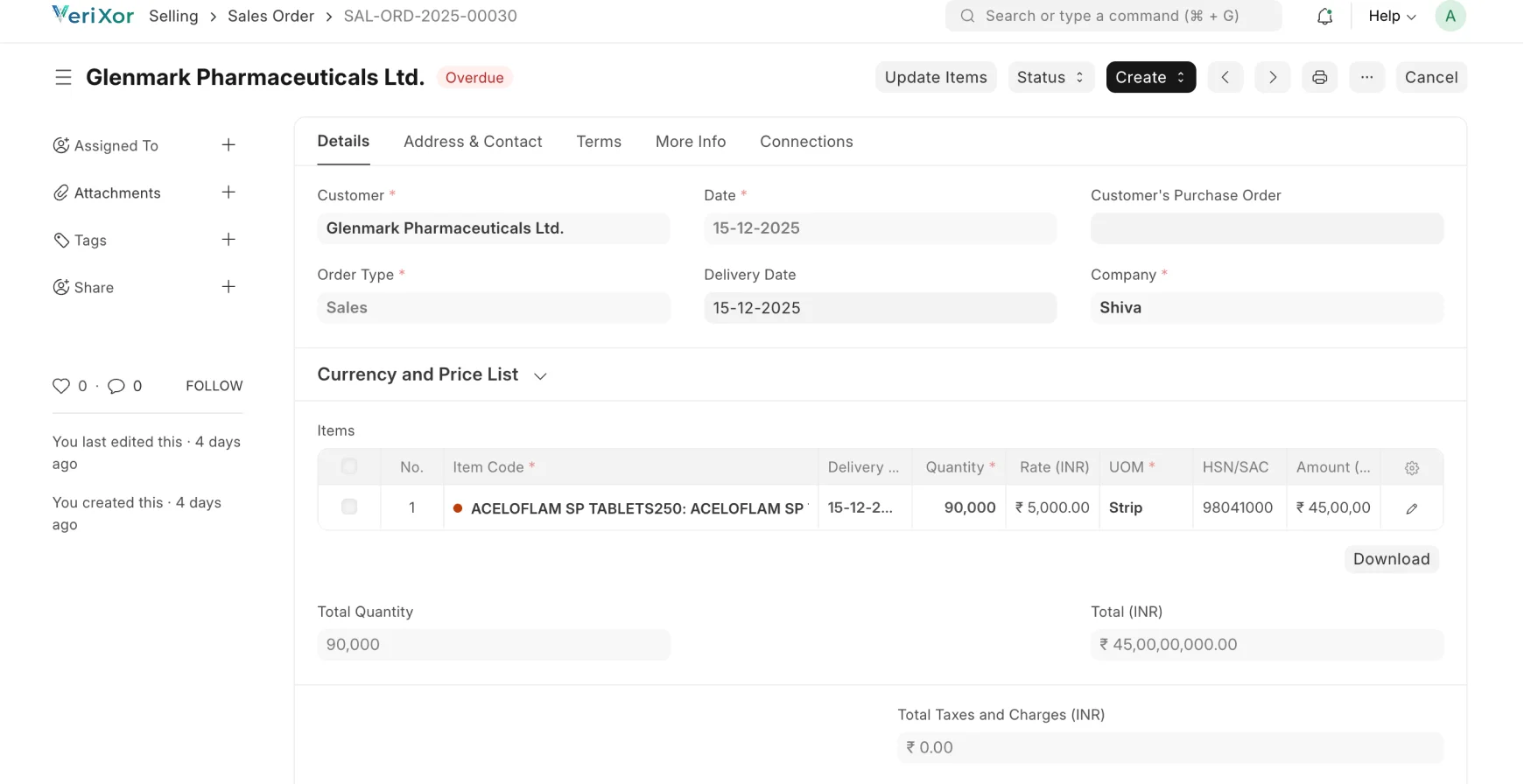
Planning & Batch Scheduling
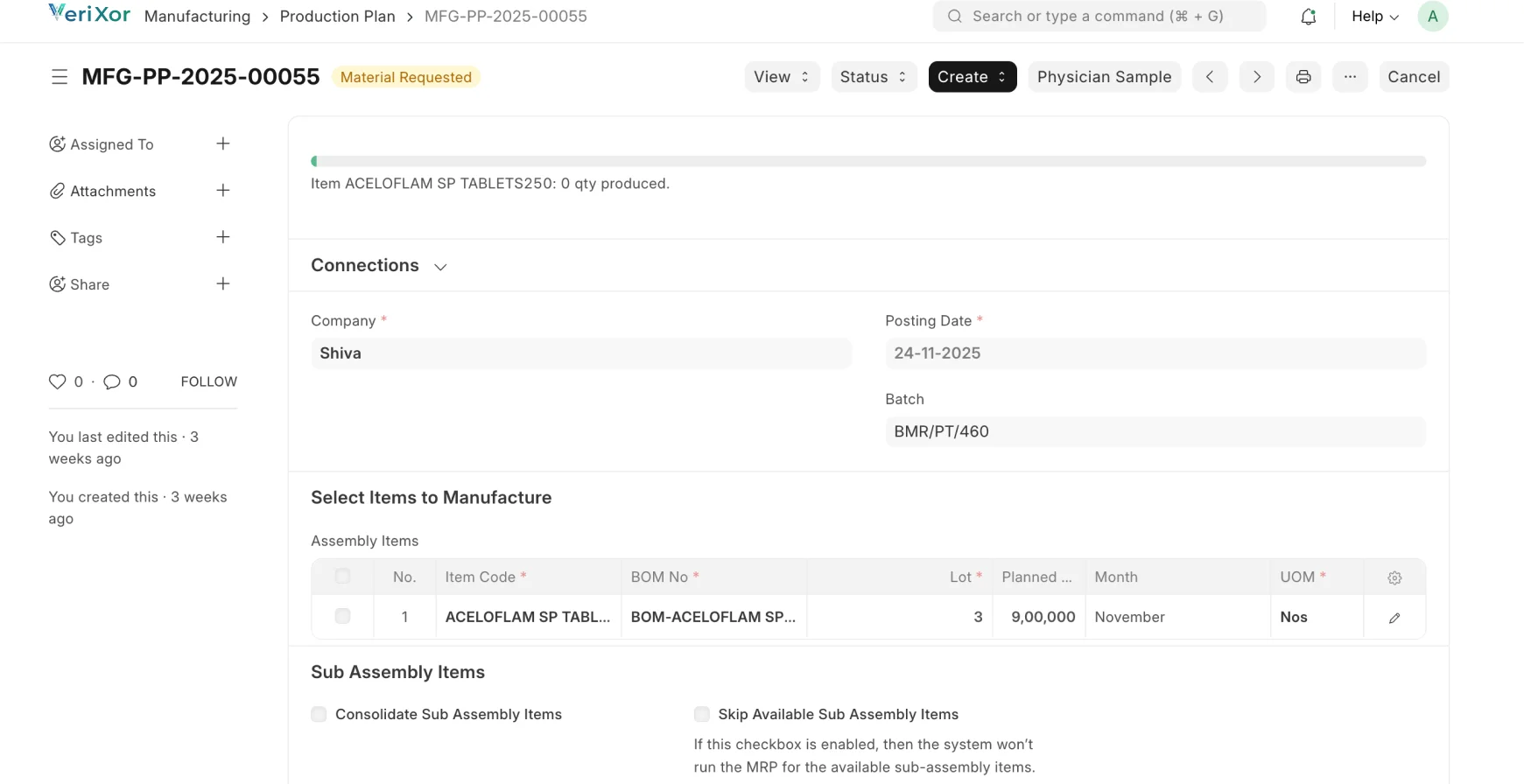
Procurement & Material Control
Batch Manufacturing & QA
Batch Release & Dispatch
Looking for a GMP-ready, compliant ERP for
Pharmaceutical Manufacturing?
Go LIVE in just 2 weeks and gain full control over batches, quality, and compliance.
Run Our Manufacturing ERP with Your Existing Financials
Integrate. Extend. Excel.
Proven At Scale
VERIXOR ERP manages every core pharmaceutical manufacturing process—from production to compliance.
Frequently Asked Questions
VERIXOR is an ERP system designed specifically for pharmaceutical companies. It helps manage formulations, R&D, batch production, quality checks, traceability, planning, warehousing, costing, and more—while staying compliant with FDA, cGMP, and 21 CFR Part 11 requirements.
VERIXOR supports regulated manufacturing with features like electronic records and signatures (21 CFR Part 11), audit trails, CAPA, non-conformance tracking, SOP control, BMR/MBR records, COAs, supplier management, and complete lot traceability—aligned with FDA and GxP expectations.
Yes. VERIXOR allows you to log non-conformance issues, manage CAPA workflows, track quality actions, and perform quality or stability testing as part of your batch and quality management processes.
VERIXOR provides secure formula management with version control, approval workflows, templates for dosage forms like capsules or softgels, label-claim tracking, formula comparison, batch scaling, physical property management, and BOM support for batch and packaged products.
Yes. VERIXOR tracks lots and expiry dates, enforces FEFO, manages quarantine and release processes, mandates SOP and QC checks, maintains full audit trails, and controls lot movement based on quality status.
Ready To
Go Live in Just 2 Weeks with

#1 Compliance-Ready ERP for the Pharma Industry
An advanced ERP platform built for every manufacturing and distribution need.
Drop Your Business Query NowA 100% Guaranteed Solution for your Organizations























