Quick Summary
Pharmaceutical quality control ensures medicines made in manufacturing are safe and reliable. It includes testing raw materials, monitoring production steps, and checking finished products. Control samples and regulations help keep quality consistent.
Table Of Contents
Introduction
For the pharmaceutical industry, since it deals with so many potent, life-saving, and important elements and products, it is very important that it follows, maintains, and strictly adheres to pharmaceutical quality control processes. This ensures that all medicines are safe, effective, and meet required standards before reaching patients. Quality control in the pharmaceutical manufacturing process plays a critical role in protecting patient health and meeting legal regulations. In this blog post, we will explain how quality control is applied in pharmaceuticals and why it is essential for producing reliable medicines.
Key Takeaways
Pharmaceutical quality control ensures medicines are safe and meet required standards.
Testing starts with raw materials, continues during production, and ends with final product checks.
Control samples help verify product quality and investigate issues if needed.
Meeting regulatory rules like FDA and EMA standards is essential for compliance.
Proper documentation and staff training support consistent quality and regulatory audits.
What is Quality Control in Pharmaceutical Manufacturing?
Let us understand what actually is managed and maintained when it comes to quality control in pharmaceutical manufacturing. It is a process that checks and tests the pharmaceutical products at different stages to ensure they meet quality standards. This includes testing raw materials, monitoring the manufacturing steps, and examining the finished products.
And here the goal is to make sure medicines are safe, effective, and pure, and that’s why it is important to know that quality control focuses on testing and inspection, while quality assurance covers overall system and process management. Together, they help produce reliable medicines by controlling all stages from raw materials to finished products in the pharmaceutical manufacturing process.
The Main Steps in Pharmaceutical Quality Control
There are some important, required, and basic steps in pharmaceutical quality control to ensure product safety and effectiveness.
Raw material testing: This step involves checking the identity, purity, potency, and contaminants of all materials before they enter the pharmaceutical manufacturing process, which also prevents poor quality inputs affecting the final drug.
In-process quality control: Here, IPQC means monitoring the manufacturing steps live to catch any changes or problems, such as temperature or pH shifts, that could affect quality.
Finished product testing: This important stage includes detailed tests to confirm the drug’s strength, stability, and safety before it is released for use.
Role of control sample in pharma: The control sample in pharma is a stored sample from each batch used later for testing or to resolve any quality issues.
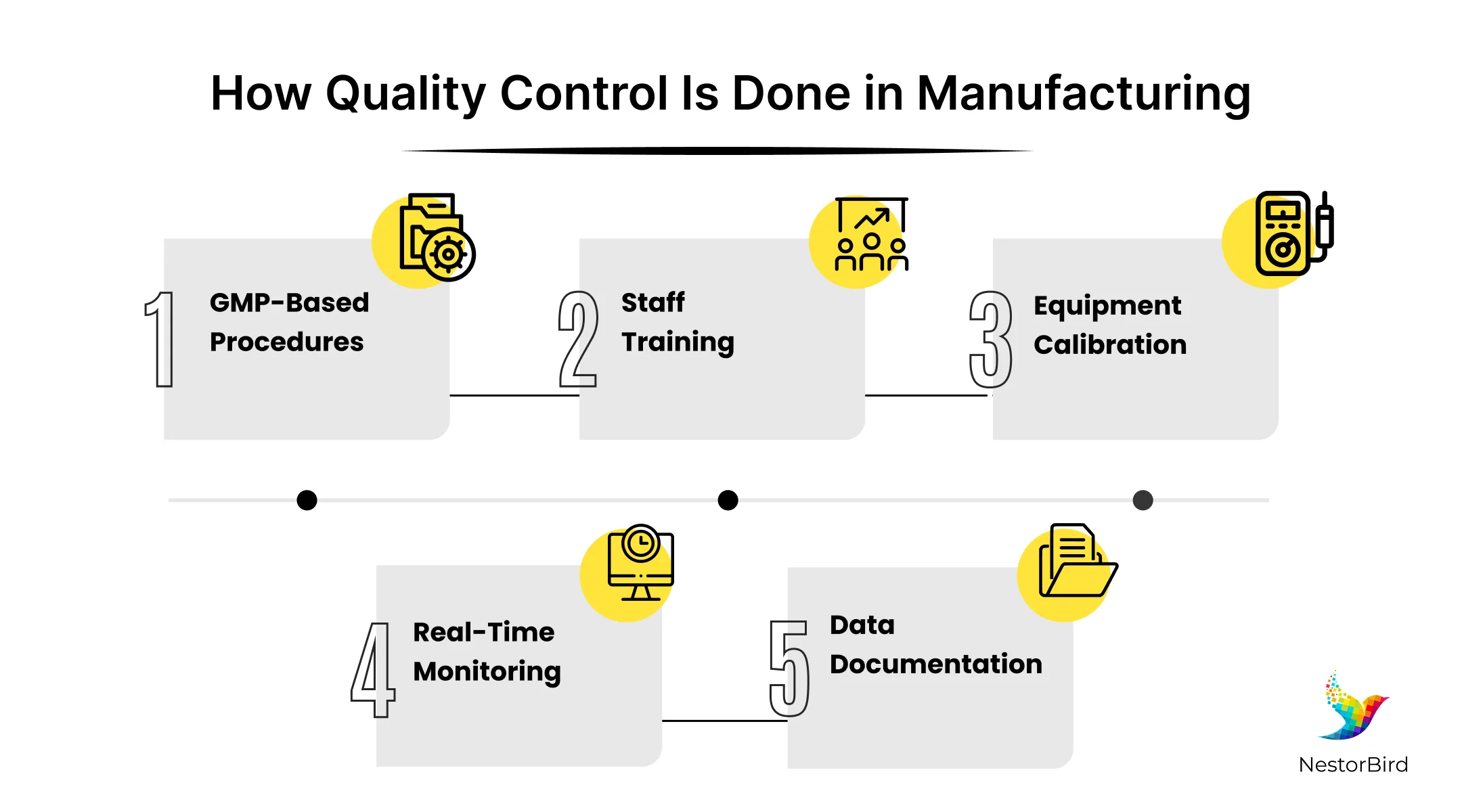
How Quality Control is Implemented During Manufacturing
During the main manufacturing phase or stage of the various potent pharma products, quality control in pharmaceutical manufacturing is carefully integrated with good manufacturing practices to ensure consistent quality.
Integrated QC practices with good manufacturing practices: All manufacturing steps follow clear, validated procedures, where every process is documented to ensure that products meet quality standards and meet legal and safety requirements.
Continuous quality control: Equipment is regularly calibrated, and staff receive proper training to carry out tasks correctly, and this constant monitoring helps detect and fix problems early to avoid defects.
Proper statistical and automated control methods: The different data and automated systems are used to track product quality, which also helps prevent errors and improves product reliability throughout the pharmaceutical manufacturing process.
This is How ERP Enhances Efficiency Compliance Pharma.
The Importance of Control Samples in Pharmaceutical QC
The control samples have an important and useful role in maintaining and verifying quality in pharmaceutical products. These samples include raw materials, intermediate products during manufacturing, packaging materials, and finished products. Each type is carefully collected and stored following strict regulatory guidelines to ensure their condition remains unchanged.
And it is these control samples that help in future investigations if any quality issue arises. They allow manufacturers to compare problematic batches with original samples to identify the cause. These samples also help in managing customer complaints by providing evidence for quality checks.
So here, proper handling of control samples ensures the integrity of products throughout the pharmaceutical manufacturing process and supports consistent pharmaceutical quality control.
This is How ERP Systems Help Pharmaceutical Industries.
Challenges and Solutions in Pharmaceutical Quality Control
Let us understand what are some of the factors that can compromise or lead to poor quality control in pharmaceutical manufacturing.
One common cause is process deviations, which happen when the manufacturing steps do not follow the set procedures. Another cause is using contaminated or low-quality raw materials. Also, untrained or inexperienced staff may make mistakes that affect the product quality.
Poor quality control can be dangerous because it may produce medicines that are not safe or effective. It can also damage the reputation of the manufacturer and cause legal problems.
To fix these problems, manufacturers improve their pharmaceutical manufacturing process by validating all steps to make sure they work well. They provide good training to their workers and keep equipment well-calibrated. Regular audits check the whole system and help find issues early. These solutions help produce safer and better medicines.
These are the ERP for Pharmaceuticals Challenges and Benefits.
The Regulatory Standards and Compliance Required for Quality Controlled in Pharmaceuticals Manufacturing
As we all know that the pharmaceutical industry is a very highly regulated industry as it has to ensure the safety and effectiveness of medicines. Key regulations guiding pharmaceutical quality control come from agencies like the FDA (U.S. Food and Drug Administration), EMA (European Medicines Agency), and WHO (World Health Organization). These agencies set strict rules for how medicines should be made and tested.
So now, companies must align their quality control in the pharmaceutical manufacturing process with these regulations. They do this by following Good Manufacturing Practices (GMP), performing regular quality checks, and training their staff properly.
Here, the proper and valid documentation using pharma supply chain software plays a very important role, where every step of manufacturing and testing is recorded in batch records. These records help in verifying quality and provide proof during inspections and audits. Keeping detailed records ensures transparency and accountability in pharmaceutical production.
Final Note
Using a powerful pharmaceutical quality control is very important throughout the entire pharmaceutical manufacturing process and dictate how quality control in pharma works. It ensures that medicines are safe, effective, and consistent in quality. Testing at different stages and using control samples in pharma help maintain reliable standards. Using ERP solutions from NestorBird can make managing quality control easier. These systems help track processes, store important records, and keep everything organized. This supports faster decision-making and better compliance with regulations during pharmaceutical manufacturing.
Frequently Asked Questions
Quality control helps ensure medicines work as intended and are safe for patients. It also helps companies follow the law and prevent bad products from reaching the market.
Control samples are saved from batches to test later if issues arise. They help investigate problems and compare product quality over time.
Companies follow rules set by agencies like FDA, EMA, and WHO by using good manufacturing practices, detailed testing, and keeping proper records.
If a test fails, the batch is investigated to find the cause. Corrections are made, and the batch may be rejected if it doesn’t meet standards.
Automation, statistical analysis, and real-time sensors help monitor production closely and prevent errors for better product quality.