Quick Summary
An FDA validated ERP solution is vital for pharmaceutical companies to meet strict regulations and ensure product quality. This software from NestorBird helps manage compliance, control production, and improve efficiency with ease.
Table Of Contents
Introduction
The pharmaceutical industry has to follow and comply with required important regulations and rules that govern the segment, as it mainly deals with the sensitive health and safety of patients. These regulations are complex and constantly changing, making it challenging for companies to stay compliant. An FDA validated ERP solution plays a critical role in helping pharmaceutical companies manage these regulations efficiently while maintaining high-quality standards. In this blog post, you will learn how such solutions solve core challenges related to FDA compliance ERP and pharmaceutical quality management, ensuring smoother operations and regulatory adherence for pharma businesses.
Key Takeaways
An FDA approved ERP system helps meet strict pharma rules easily. It keeps steps clear and data safe.
It can save time and money by automating more work and reducing errors.
The system can track raw materials to finished products for full traceability.
It helps companies stay ready for audits with detailed records and reports.
As rules change, the right ERP can grow and adapt with the business.
Understanding FDA Validation in Pharmaceutical ERP
Let us begin by understanding what actually the FDA validation means for this industry when it comes to using the right and best suitable ERP system. FDA validation of an ERP system means proving that the software works as it is supposed to and meets important regulatory rules, which also makes sure that the system can keep accurate and trustworthy records, which is very important in the pharmaceutical industry.
The ERP solution validation process involves careful testing of the software functions, security, and reports before it is used in real operations, and this validation helps align the software with strict FDA regulatory standards, making sure the company is ready for audits and inspections without any compliance issues.
This is How ERP Enhances Efficiency Compliance Pharma.
Main Useful Features of an Effective ERP Solution for Pharma Manufacturing
The features of a FDA validated ERP solution for your pharmacuetical business can greatly help you efficiently run your business in a smooth manner and help you grow and scale too. These features are helpful to meet strict regulatory standards and pass audits easily while maintaining high product quality and safety.
Proper and Efficient Batch Records and Recipe Management: This feature keeps a detailed record of every batch made in the pharma plant, and also tracks the ingredients, steps, and formulas used. This helps maintain consistency and quality. It is a key part of GMP compliant ERP systems.
Use of Electronic Signatures and Audit Trails: The system records electronic signatures to approve workflows, making it easy to trace actions and changes. Audit trails help during inspections, and these features help the pharmaceutical ERP software to meet the necessary legal standards.
Efficient Automated Quality Management: This is important for this industry, and the feature automates quality checks, testing, and issue tracking. It reduces human errors and helps maintain documented proof of quality processes. Having automation can support effective pharmaceutical quality management and regulatory compliance with ERP for pharma manufacturing.
Choose the best Inventory Management Software in Pharma Manufacturing.
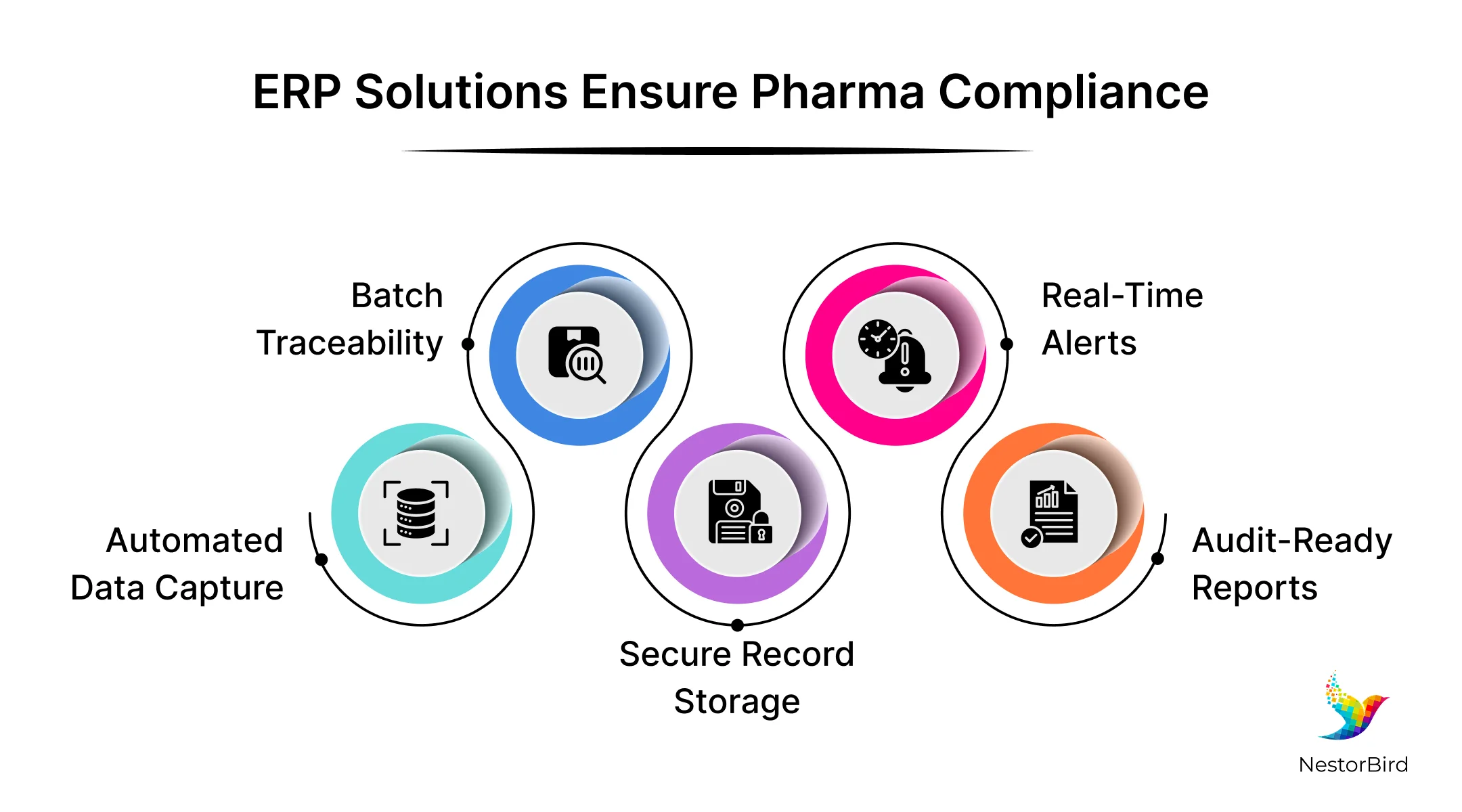
ERP solutions Ensure Compliance with Pharmaceutical Industry Regulations
When you implement the right FDA validated ERP system for your pharma business, it helps you follow important rules like FDA 21 CFR Part 11 and cGMP. These rules control how companies manage electronic records and signatures safely and legally. The ERP system must keep accurate data and show who changed what and when. This helps the company stay ready for FDA audits and inspections.
The ERP system also supports pharmaceutical supply chain management by tracking raw materials, production, and finished products. It helps manage recalls quickly by tracing items through the supply chain. This makes the system reliable and safe.
Here, maintaining FDA compliance in pharma is very important during fast changes in manufacturing and supply chain. The ERP system helps companies respond to these changes without losing control of quality or data. It keeps everything organized and up to date. This way, companies avoid costly mistakes and stay trustworthy with regulators and customers.
This is how you can Improve Batch Processing With Pharma ERP Software.
Challenges Solved by a Pharmaceutical ERP Software
The pharma business usually deals with a few challenges when it comes to managing the different operations of the business, while also staying compliant, and using the best suitable ERP software can really help businesses solve those issues effectively.
It can reduce manual paperwork by automating batch documentation and creating audit trails. The system can save time and lower errors, so when the business implements it for their business, it can meet FDA compliance ERP rules more easily.
The system can keep data safe and correct by using strong security controls. It can follow the strict rules set by FDA validated ERP. If the business protects data well, it avoids problems with regulators.
It can make complex validation simple by giving tools to test and watch the system over time. The system can help with ongoing compliance tasks, and when you use these tools, your business stays on top of the rules.
The system can manage many sites and help check suppliers in one place. If the business uses this, it improves how locations work together, as it also supports pharmaceutical software solutions to keep quality high everywhere.
Learn How can Pharma Manufacturers Manage Rapid Production.
Benefits of Implementing an FDA Validated ERP Solution
It can help improve FDA compliance in pharma by ensuring all processes follow regulatory rules. The system can reduce risks by keeping data accurate and complete.
The system can make operations faster and cost less by automating tasks and reducing manual work. If the business uses this, it gets better pharmaceutical ERP software efficiency.
It can give clear views of the whole supply chain and production steps. The system can track raw materials to finished products. This helps control quality and delivery on time.
The system can grow with the business by adjusting to new rules and more users. It stays up to date with changing FDA regulatory standards so the company stays compliant.
It can help manage risks better by giving tools to check quality and fix problems early. This supports strong pharmaceutical quality management and lowers chances of costly mistakes.
End Note
Using an FDA validated ERP solution helps your pharmaceutical business follow important rules easily. Using ERP solutions from NestorBird is a smart choice because they build software just for pharmaceutical companies. The systems help you stay compliant, work smoothly, and grow with real-time data and strong support, which makes us a reliable partner for your pharma businesses.
Frequently Asked Questions
FDA compliance ensures that pharmaceutical products are made safely and meet quality standards. It avoids penalties and keeps the company trusted by customers and regulators.
ERP system validation proves the software works as needed for pharma use. It involves testing functions, security, and data accuracy to meet FDA regulatory standards.
Yes, an ERP system can track raw materials, suppliers, inventory, and finished products. This improves traceability, helps manage recalls, and keeps the supply chain efficient.
Yes, ERP systems can manage operations across many sites in one platform. They help coordinate production, suppliers, and quality controls smoothly.
It reduces risks of errors, data loss, and non-compliance. The system keeps data complete and secure, helping pharma avoid penalties and product recalls.