Quick Summary
This blog explains how the pharmaceutical manufacturing industry can handle fast production while keeping high safety and quality standards. Using a good quality management system and pharma ERP software helps companies meet rules and make better products safely.
Table Of Contents
Introduction
There can be bouts of time frames where due to any reason there is a sudden urge to manage and produce large batches of pharma products, and then the pharmaceutical manufacturing industry must work very fast but still make safe, high-quality medicines. These companies always follow strict rules from health authorities and must keep the product quality very high. Often, increased demand, supply chain delays, or safety checks can make production harder. In this blog post, simple steps and tips for handling these challenges and meeting high standards in rapid pharma production will be shared.
Key Takeaways
A strong quality management system helps ensure safe, high-quality medicines in the pharmaceutical manufacturing industry.
Pharma ERP software improves production speed and keeps data accurate while meeting compliance needs.
Quality control checks for impurities and prevents problems during fast production stages.
Managing product quality complaints quickly helps improve medicines and customer trust.
Combining ERP and quality systems supports steady production and reliable quality assurance.
Problems The Pharmaceutical Manufacturing Industry Faces With Fast Production
During the rapid production phase of the potent and important pharmaceutical products, it is absolutely important to maintain high standards of safety, quality, and compliance. A strong quality management system helps control how products are made, manages risks, and follows all rules. It is more than just paperwork or audits.
So now, to build such a system, companies focus on managing the whole product lifecycle, keeping clear documents, improving processes continuously, and ensuring leaders support quality goals. In the quality management in pharmaceutical industry, flexible and fast systems are needed to keep meeting regulations and ensure every batch is safe and consistent for patients.
Learn Why Data Integrity Is Key to Pharmaceutical Compliance.
How A Quality Management System Helps Control How Medicines Are Made
The many integrated quality management system benefits: When pharma industries comply with and implement the right quality management system during production, they can better control processes, reduce risks, and meet important rules. An integrated system is not just for audits or following SOPs, as it helps keep the whole manufacturing flow smooth and safe. This is needed to avoid errors and keep products safe for patients, which is beneficial and important for the pharmaceutical manufacturing industry.
Designing an effective quality system: When designing a good QMS, pharma companies must manage the entire product lifecycle carefully, keep proper documentation, focus on improving all steps continuously, and have strong support from top management. These steps help keep everything organized and correct, making it easier to find and solve problems quickly, and such systems improve overall reliability and efficiency in pharmaceutical manufacturing.
Efficient systems for quality control: The quality management in pharmaceutical industry needs to be flexible and quick to adapt because rules and conditions change. Agile systems help companies follow compliance and keep product quality consistent even when producing fast or scaling up. This means pharma manufacturers can keep making safe, good products every time without delays or risks.
Learn more about ERP for Pharmaceuticals Challenges and Benefits.
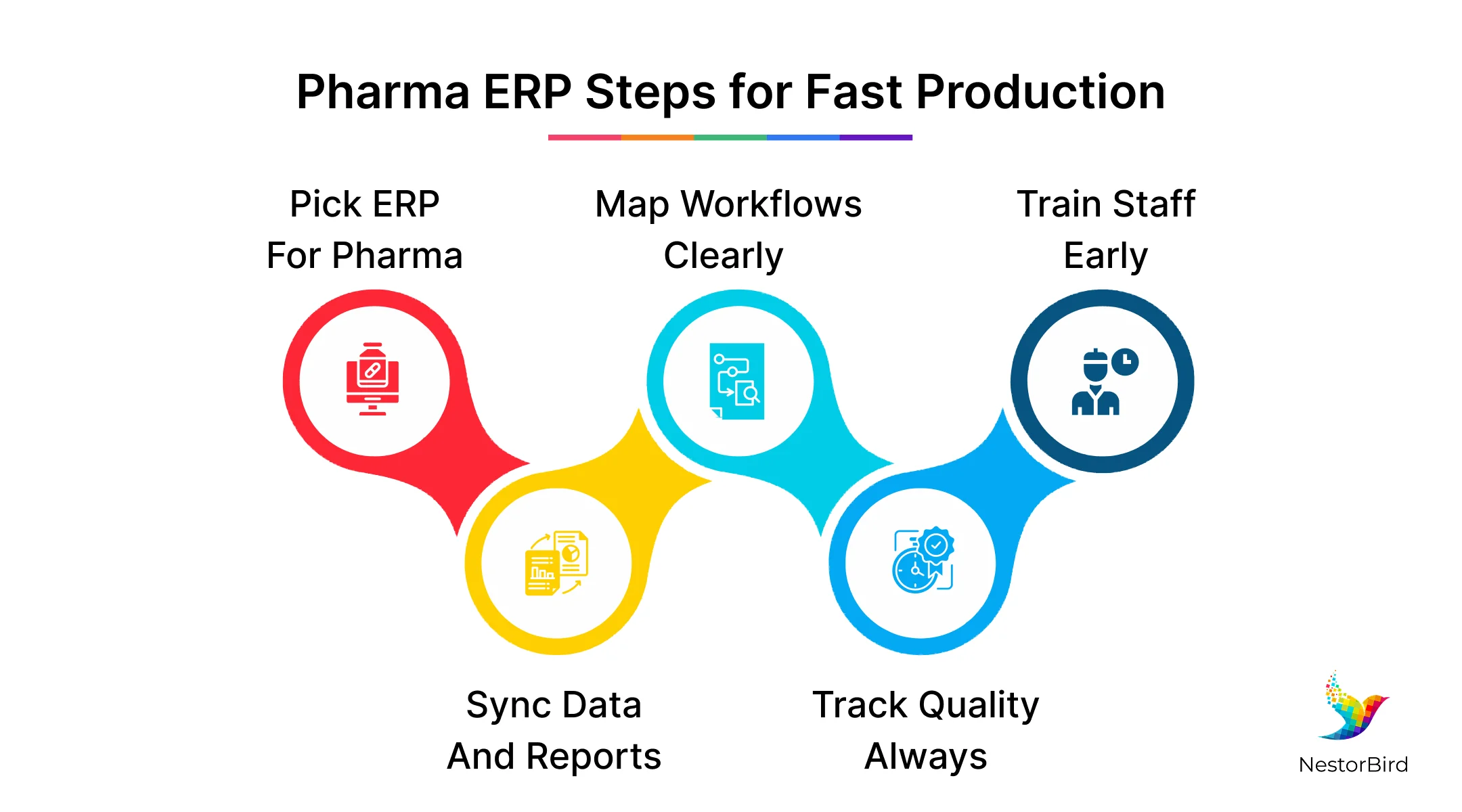
Using and Implementing Pharma ERP Software to Effectively Manage Rapid Processes
When pharma companies use an efficient and the right suitable pharma ERP software, they can speed up production while keeping data accurate and following rules. This software helps organize work in real time, making sure everything from production steps to quality checks is tracked well. The pharmaceutical manufacturing industry uses ERP modules to control batches, manage formulas, and create reports that meet government standards.
But there are many challenges when introducing ERP, like handling changes in staff habits, updating old systems, managing supply delays, and validating the software for strict pharma rules. Despite these challenges, effective use of ERP can make production smoother, reduce errors, and help companies meet both speed and quality needs in their processes.
Advanced Quality Control and Assurance Practices For The Industry
Why strong quality control is needed: If there is a rapid process of production, it is very common that, quality control is very important at every step. It helps check for impurities, track quality in real-time, and stop problems before they grow. This ensures the medicines are safe and meet all quality rules.
Following more than just basic compliance: Companies should understand that to manage pharma quality assurance is more than just following rules, and it helps reduce risks, cut costs, and improve production all the time, and it is this ongoing improvement that makes medicines safer and helps companies work better over time.
Managing quality in big production: In large factories, managing all aspects of quality management is important, which mainly includes validating equipment, doing audits, and managing suppliers carefully. These actions make sure production stays steady and meets all quality standards.
This is How ERP Enhances Efficiency Compliance Pharma.
Efficiently Managing Product Quality Complaints & Continuous Feedback
When the production process is sped up during rapid production, it can happen that there are some minor problems with product quality. To handle this well, pharma companies use structured workflows to manage product quality complaints. They keep digital records, apply Corrective and Preventive Action, and ensure open communication, which also helps to track and resolve issues quickly and clearly.
That’s why feedback from product complaints is important for improving quality. Pharma companies use this feedback to strengthen quality management in pharmaceutical industry. They find the root causes of problems and provide retraining to staff. This continuous process helps prevent future issues and supports ongoing improvement in making safer medicines.
The Best Practices for Managing Rapid Production Processes
You can use data and analytics to monitor production and fix issues quickly, as this helps pharma manufacturers keep both speed and quality high during fast production times.
It is good practice to manage risks before they become problems by planning and acting early, which keeps products safe and production smooth.
Companies need to train workers regularly so they know how to keep quality high even when production is fast or changing.
It is best to use flexible supply chain methods that can adjust to changes quickly. This helps avoid delays and keeps production steady.
When you are planning to grow and scale your business, combine ERP and quality systems to keep quality assurance strong during growth or when moving to new production methods.
Let us see How ERP Systems Help Pharmaceutical Industries.
Conclusion
When managing the production processes well, pharma manufacturers should use advanced data tools to monitor processes and fix issues fast. Planning ahead helps reduce risks and keeps production safe and efficient. Using ERP solutions from NestorBird can help pharma companies stay compliant and work smoothly, as the ERP software is easy to use and built for pharma needs, helping maintain quality and meet rules without extra hassle, which makes production faster and safer while supporting growth.
Frequently Asked Questions
It checks for impurities and fixes problems quickly to keep batches safe and consistent in fast pharma manufacturing.
By using clear workflows, digital tracking, and corrective actions, companies quickly solve issues and improve product quality.
ERP manages resources and operations, while QMS focuses on ensuring quality—together they make production reliable and compliant.