Quick Summary
Validation software for pharma helps ensure medicines are safe and meet strict quality rules. This software supports compliance with regulations and improves production reliability. Using ERP system validation tools reduces risks and simplifies audits for pharmaceutical companies.
Table Of Contents
Introduction
Since the pharmaceutical industry is expected to comply with numerous strict and necessary regulations and compliances, it is important that every step in making medicines is correct and safe. Today, making medicines is more complicated because companies must handle new formulas, technology, and strict quality rules. In this blog post, the need for validation software for pharma is clear, as it helps companies meet all safety and regulatory demands. Without proper validation, it is hard to keep products safe and reliable for patients.
Key Takeaways
Validation software ensures medicines meet safety and quality standards.
It helps pharma companies follow strict rules and avoid fines.
ERP system validation supports smooth operations and keeps data accurate.
Automated validation tools reduce errors and save time.
Proper validation lowers risks of recalls and protects patients.
The Meaning of Software Validation in the Pharma Industry
As per the industry standards that are usually high and also strict for pharma products, validation in pharma means checking if the software works exactly as it should for making and controlling medicines. This process is different from general software testing because it is not just about finding bugs, as it makes sure the software meets special safety, quality, and regulatory rules.
Software validation in pharma includes checking that all data, records, and workflows are safe and traceable. This is why validation software for pharma is needed, and here only specialized tools can handle strict industry rules and provide the documented evidence required by agencies like FDA and EU.
That’s why using the right software validation services helps companies keep up with changing requirements and supports audits and inspections easily.
This is How ERP Systems Help Pharmaceutical Industries.
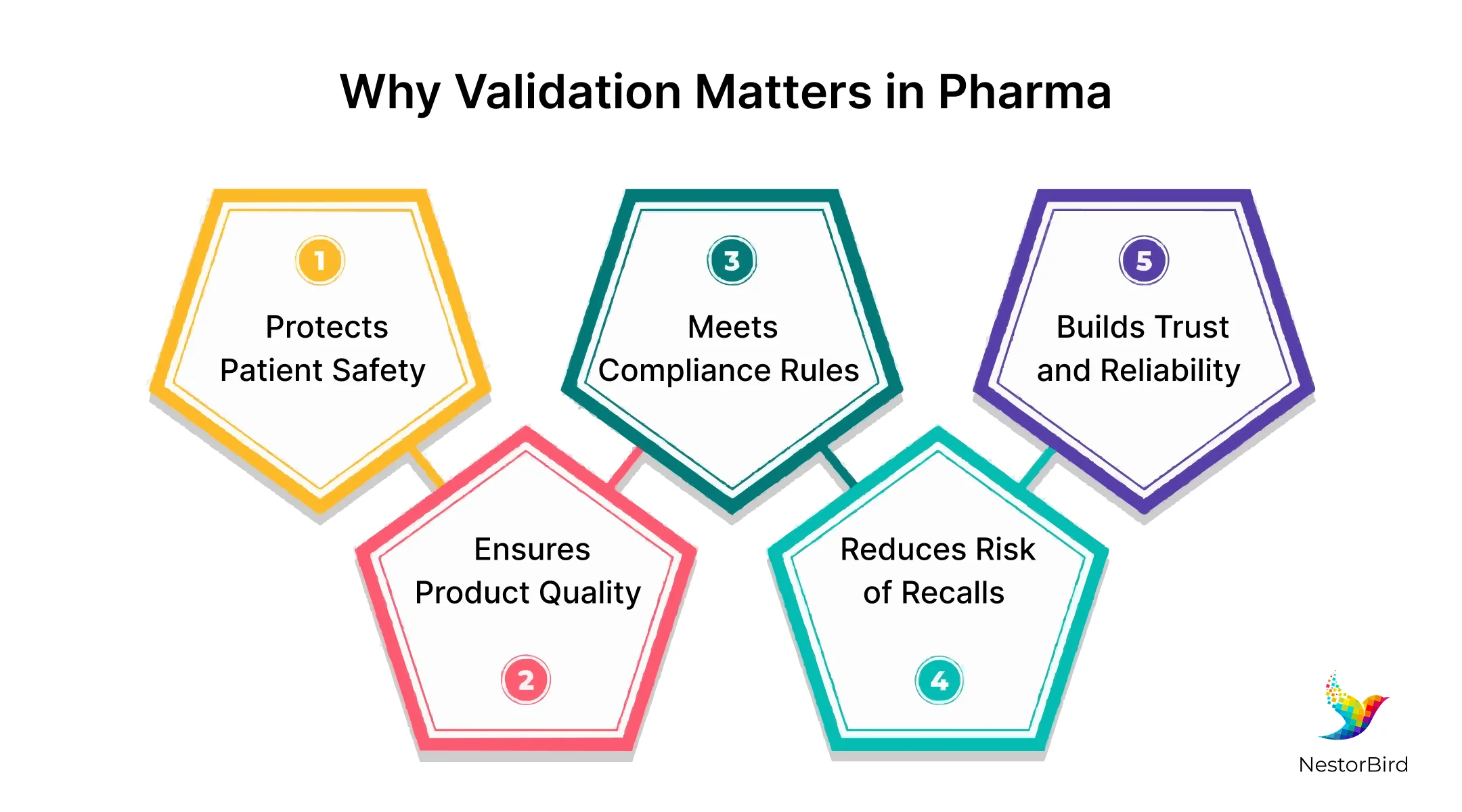
Importance of Validation in the Pharmaceutical Industry
It is important to validate numerous aspects and features of all pharma manufacturing processes to keep medicines safe for patients and follow the rules set by health authorities. When companies do not use validation in pharma correctly, they risk releasing bad products that can hurt people or cause major recalls.
Because here, failing to validate can also lead to big penalties from regulators and damage a company’s reputation. And thus, using an efficient and best suitable ERP system validation helps avoid these problems. Valid validation supports quality control, keeps production reliable, and protects everyone who depends on good medicines, which is the importance of validation in pharmaceutical industry.
Understanding the Why and How of ERP System Validation
Let us understand why ERP system validation is very important in the pharmaceutical industry. ERP systems manage key parts of pharma operations like manufacturing, inventory, quality checks, and compliance reporting. These systems must work correctly to keep data safe and products high quality, and the validation ensures the ERP system follows strict rules like FDA 21 CFR Part 11 and other global standards that may be required here.
Also, the practical steps for ERP system validation in the Pharmaceutical Manufacturing Software include creating a validation master plan, which means defining the user requirements and testing the system through installation, and then operational, and performance qualifications. Thus, the common challenges are managing complex documentation, handling changes to the system, and making sure all users follow processes correctly. Using proper ERP validation supports regulatory compliance and helps avoid risks like recalls or penalties.
A Few Important Customer Challenges in Software Validation
Pharma companies may face some challenges during the validation of the software, and let us understand some of them.
Limited Available Resources and Skills: Many pharma companies struggle to find enough trained staff for software validation, and then hiring experts for software validation services can be costly and time-consuming. Without skilled teams, the validation process may be slow or incomplete, causing delays and risks.
Managing the Complexity of System Integrations: Pharma systems like ERP, LIMS, and MES can usually need to work together, so integrating these systems can be difficult and create errors. Managing this is a common pain point when using validation software for pharma.
Keeping Up with Numerous Regulatory Changes: Pharma regulations change regularly worldwide, and then staying updated and adapting validation processes consumes time and effort. So, companies must continually revise their validation to avoid any kind of non-compliance penalties.
Changing Ongoing vs One-time Validation Needs: Validation is not a task you do just once, and many companies find it hard to keep up with validation as they deal with new updates and checks. Ongoing validation takes more resources, but it helps ensure compliance and keeps the product safe.
Learn more about pharmaceutical quality control.
How Validation Software for Pharma Solves Real Problems
With so many challenges that we saw previously, pharma companies can use validation software for pharma to reduce risks and improve operations. Case studies show that proper software validation services help companies follow rules better and prepare easier for audits. This lowers the chances of costly mistakes or recalls.
Also, the regular use of these automated validation tools can cut down manual work, reduce human errors, and save time in documentation. This helps companies finish validation faster while keeping data safe, and also lowers costs by standardizing different processes and reducing any repeated work. This way, proper validation maintains product quality and protects patients effectively.
This is How ERP Enhances Efficiency Compliance Pharma.
How To Choose the Right Validation Software for Your Pharma Company?
Check Proper and Required Regulatory Compliance: It is best to choose software that meets pharma rules like FDA and EU regulations. This helps stay legal and avoid fines, and make sure it supports ERP system validation needs and keeps clear records for audits.
Ensure Validation Software Ease of Use: You should pick software that is easy for your team to learn and use, as this mainly reduces errors and speeds up validation work. User-friendly tools support better software validation services and improve team efficiency.
Check for Flexibility and Scalability Features: The software should adapt to changes and grow with your company. It must handle updates without too much rework, which also helps keep validation processes current and compliant over time.
Check for Automation Features: Automated testing, reporting, and documentation reduce manual work and errors, so choose a software with these features that saves time and cuts validation costs while improving accuracy.
Should Provide Strong Support and Documentation: You should select vendors who provide clear validation templates, training, and technical support, as good support helps follow the right steps and resolves issues quickly during validation.
Conclusion
Validation in pharmaceutical manufacturing is very important to keep medicines safe and meet strict laws, and using the right software makes this work easier and faster. Choosing ERP solutions from NestorBird is a smart choice because the software is made specially for the pharma industry. It helps companies stay compliant with regulations and organizes all processes from production to quality control in one place.
Frequently Asked Questions
It is the process of making sure software used in pharma works correctly and follows all rules to keep medicine safe and reliable.
It reduces human errors, speeds up audits, documents processes, and helps companies follow strict quality and safety rules.
Experts ensure software meets all standards, reduce risk, and save time compared to in-house validation with limited skills.